Testing for infectious diseases such as malaria, HIV, dengue, etc., and non-communicable diseases such as heart problems and diabetes requires a good tool for accurate analysis. Previously, diagnostic procedures like mass spectroscopy demanded expensive laboratory setups and were time consuming. These devices also required extensive cleaning procedures and skilled professionals for performing the tests.
Thus, the authors of this paper, which include Lalitha Pratyusha Bheemavarapu, Dr. Jayaraj Joseph, and Prof. Mohanasankar Sivaprakasam from the Department of Electrical Engineering, Indian Institute of Technology Madras, Chennai, India, and Malay Ilesh Shah from the Healthcare Technology Innovation Centre, Indian Institute of Technology Madras, Chennai, India, have developed lateral flow immunoassays (LFIA). These are simple to use diagnostic tools, disposable, and they provide efficient results quickly. The main parameters affecting the performance of lateral flow immunoassays are:
1. Good reproducibility
2. Accuracy
3. Sensitivity
Manufacturers need to take care of multiple factors to meet these requirements. The capillary flow rate of the sample through the membrane forms an important parameter for test strip design.
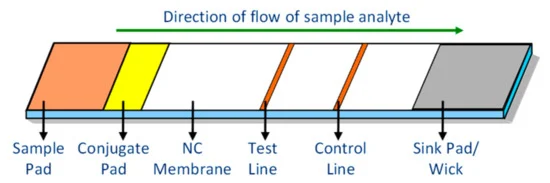
The parameters must be constant for all the test strips manufactured. However, the manufacturing tools and processes could account for a reduction in strip-to-strip reproducibility.
Thus there is a necessity for practical LFIA kit evaluation tools that assist the manufacturers to determine the performance of test strips.
An image-based visualization tool assisting the quantitative LFIA kit manufacturers towards the development of high-performance test cartridges is presented. The developed tool is compatible with fluorescence labelled LFIA cartridges. It provides a practical analysis of sample flow properties, including capillary flow rate, practical location of the test, and control lines. It can be used to practically evaluate the optimum reaction time.
The hardware setup consists of a camera sensor-based fluorescence reader. There are no moving components in the hardware setup.
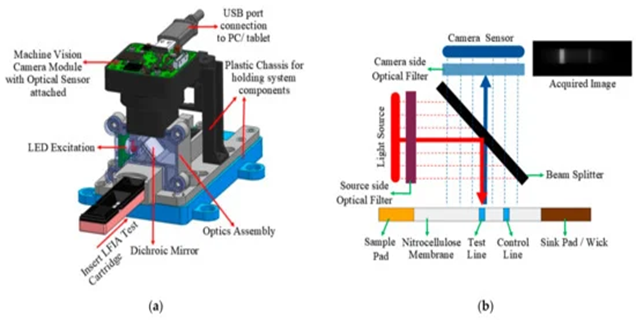
With a fixed sensing and excitation mechanism, the system aids in studying the reaction progress of the sample under test, right from the time the sample is dispensed onto the test cartridge. The proposed tool is compatible with fluorescence-labelled quantitative lateral flow assays. Image processing-based algorithms were developed to track reaction progress of the sample. The designed tool is practical and it examines the performance of manufactured test strips.
This tool brings down the level of human intervention and thereby provides a reliable, practical solution for increased automation in the field of medical diagnostics. Evaluation of the algorithm was done with Glycated Hemoglobin (HbA1C) and Vitamin D test cartridges. The reaction stability time of HbA1C test samples was measured to be 12 minutes, and that of Vitamin D samples was at 24 minutes. Sample flow abnormalities were detected at 100 percent accuracy, and the membrane irregularities were detected at 96 percent accuracy thus making this study quite successful.
Dr. Shalini Gupta from the Department of Chemical Engineering, Indian Institute of Technology Delhi, New Delhi, India, gave the following views on this study: “LFAs may be conceptually simple devices but they have a complex architecture that requires iterative evaluation of multiple critical parameters during the development and manufacturing stage. The quantitative imaging tool IQVision presented by Jayaraj and team is a unique platform that helps improve the analytical performance and reliability of LFAs through real-time data acquisition of flow and flow-related abnormalities.”
Article by Akshay Anantharaman
Here is the original link to the paper:
https://www.mdpi.com/2079-6374/11/7/211