Reba McEntire says,“To thrive in life you need three bones; a wishbone, a back bone and a funny bone”. However, we all know in a healthy adult human body, we have 206 bones.From helping us to do physical activities such as standing, running, jumping and skipping etc. to protecting our vital organs like heart and lungs, bones play more important roles than just being the scaffolds that shape our body. Be it the smallest bone -stapes- or the longest bone-femur-, each of the 206 bones in our body has their own important tasks that allow smooth functioning of the body. Mainly made up of protein collagen, calcium and minerals, they are indeed the strong pillars on which the human body stands straight and moves around. Though they have an excellent inherent repair capacity, they lack the ability to bridge large bone defects on their own. Therefore, major bone injuries due to accidents or any other ailment can be devastating as they restrict the mobility of a person for a longer duration or confine them to bed for their whole life.
With more than 2 million bone grafts being performed every year, bone regenerative procedures are on the rise across the globe. Bone defects can occur due to accidents, trauma, congenital anomalies, tissue resection due to cancer etc. are treated through bone grafting. Bone grafting, to fix the defect in the bone, is being done using autologous grafts, allografts or synthetic biomaterials. In autografts, a person’s own bone is harvested and used for sealing the defect, whereas in allografts, someone else’s bone is harvested and used for treating the defect. Although autografts remain the gold standard of bone grafting,sites from which the bone can be taken may be limited or it may happen that the patient’s body site from which the bone is taken for treatment may not heal. These issues have given a major push to the field of synthetic bone biomaterials where materials such as hydroxyapatite, calcium phosphate, titanium etc. are being used for filling the bone gaps. Prof. Mukesh Doble and colleagues at IIT Madras are working in the area of synthetic biomaterials and have been exploring the materials and ways by which the bone defects can be treated.
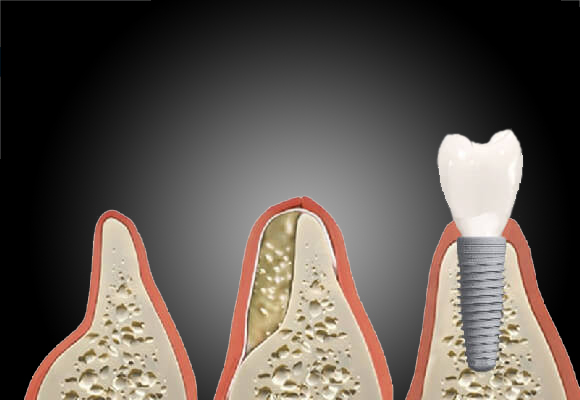
“Bones such as the fore-arm, back-arm, leg and thigh which are almost 1 foot long are called long bones in the body. Small fractures or defects can be treated with plates and rods. Fractures longer than 5 cm are called long bone segmental defects which take a long time to heal and need some sort of support. Titanium mesh cage is placed in the gap which helps to heal and stabilize the bone. But it does not degrade and so remains in the body permanently. It also leads to stress shielding since it has mechanical strength much larger than the bone,” explains Prof. Mukesh Doble, Professor at IIT Madras who leads the research group.
In their latest research, which has been published in the international peer-reviewed journal Nanomedicine, the team has shown that the nano-coated magnesium alloys can repair the bone defects in rabbits and hence its medical application in other animals and repairing human bones can be studied in more detail.
Alloys of magnesium are being considered as a good option for orthopedic applications as magnesium is biocompatible, biodegradable and has other important mechanical properties. Magnesium, infact, is the fourth abundant metal in the body and is known to accelerate the healing of bones. However, the use of magnesium alloys in bone repair is restricted due to few issues.
“Magnesium is now being looked at as an option as it can degrade slowly and reabsorbed. Its mechanical properties are also closer to that of the bone. The main problem with magnesium is that it degrades fast. In order to slow it down and match to the growth of the new bone we have coated (electrospinning) it with a slowly degrading polymer called polycaprolactone and hydroxyapatite. The latter is the same as the bone material; so it doesn’t cause any toxicity and integrates with the bone,” adds Prof. Doble.



For this study, the team used AZ31 alloy of magnesium and used it for developing magnesium mesh cage implants. Further, they coated these implants with polycaprolactone and nano hydroxyapatite by dipping and electrospinning. This nano-coated magnesium mesh was then used to heal the bone defect in femur of rabbits. The researchers found that the rabbit femur implanted with the coated magnesium alloy showed bone formation and also bridged the defect region. The team emphasizes that this was possible due to the biocompatible nature of polycaprolactone and nano-hydroxyapatite which ensured the good recovery without any adverse reactions such as fibrosis.
For this study, the team used AZ31 alloy of magnesium and used it for developing magnesium mesh cage implants. Further, they coated these implants with polycaprolactone and nano hydroxyapatite by dipping and electrospinning. This nano-coated magnesium mesh was then used to heal the bone defect in femur of rabbits. The researchers found that the rabbit femur implanted with the coated magnesium alloy showed bone formation and also bridged the defect region. The team emphasizes that this was possible due to the biocompatible nature of polycaprolactone and nano-hydroxyapatite which ensured the good recovery without any adverse reactions such as fibrosis.
“The novelty of this research resides on the biomimetic coating generated with polycaprolactone biocompatible material and hydroxyapatite, a ceramic material naturally present at the bone extracellular matrix. As a result, a nanostructured coating is generated providing to the implant surface osteoconductive aligned structures, which may serve as highways for osteogenic cells to travel to the defect area and generate new bone there. Interestingly, in this work researchers not only characterize the generated implantable structures and hypothesize about their potential biological properties, but they also empirically assess the ability of these implants to induce higher amount of bone formation in vivo, in a preclinical critical-size bone defect model.This work opens the way to the generation of clinically useful biodegradable bone implants with improved bone formation ability due to biomimetic strategies introduced in the implant design,” says Dr. Ander Abarrategi, Principal Investigator at Spain based CICbiomaGUNE Research Institute, who is an expert in the same field.
The team is exploring funding opportunities to further test this newly developed nano-coated magnesium alloy in repairing bone defects in large animals such as goat or sheep to demonstrate the efficacy of the product and show the clinical significance of this work.
The research team led by Prof. Mukesh Doble included Dr. Govindaraj Perumal from IIT Madras; Dr. BoopalanRamasamy from Christian Medical College, Vellore; Dr. A Maya NandkumarfromSreeChitraTirunal Institute for Medical Sciences and Technology, Thiruvananthapuram; Dr. D Sivaraman from Sathyabama Institute of Science and Technology, Chennai and Dr. R Selvaraj from Bioscience Research Foundation, Chennai.
Patent details: “Magnesium based degradable implants for bone defect repair applications”. Application No:201841009503, The Patent Office Journal No. 50/2019, P.No. 6043, 13/12/2019 (India).
Article by Aditi Jain
Here is the link to the research article:
https://www.sciencedirect.com/science/article/abs/pii/S1549963420300861